CellufineTMET clean
Introduction
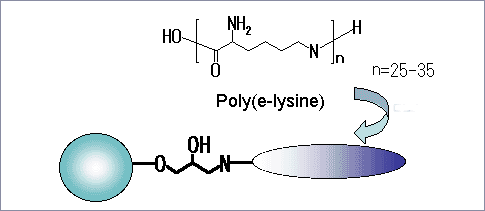
The Cellufine™ ET clean is poly(ε-lysine) immobilized Cellufine™ (cellulose spherical beads). The beads bind and remove endotoxin from your sample solution. The poly(ε-lysine) is a microbial poly(amino acid) that consist of 25-35 lysine residues produced by Streptomyces albulus. The poly(ε-lysine) as ligand and the cellulose beads act as matrix ands are products of JNC Corporation.
The Cellufine™ ET clean endotoxin removing beads were developed jointly by Kumamoto University and Chisso. The poly(ε-lysine) was immobilized onto chloromethyloxirane-activated cellulose beads. The beads are a stable affinity beads that are resistant against the cleanup solutions, which include 0.2 M sodium hydroxide and 2 M sodium
The Cellufine™ ET clean can remove endotoxin from a cellular product solution at physiological pH, ionic strength of μ = 0.02-1.0, and 0° -25C°.
Partial Structure
Characteristics
| Name | Supplied | Wet Bead Diameter | Pore Size* |
|---|---|---|---|
| Cellufine™ ET clean S | a slurry in 20 % ethanol | ca. 40-130 μm | Mlim 2000 |
| Cellufine™ ET clean L | a slurry in 20 % ethanol | ca. 40-130 μm | >Mlim 2x106 |
*The pore size (molecular weight exclution; Mlim) of the beads was estimated from calibration curves obtained by size exclusion chromatography. Pullulan and maltose were used for the Mlim determination.
Selective adsorption of endotoxin (LPS) from a bovine serum albumin (BSA) solution by Cellufine ET clean beads.
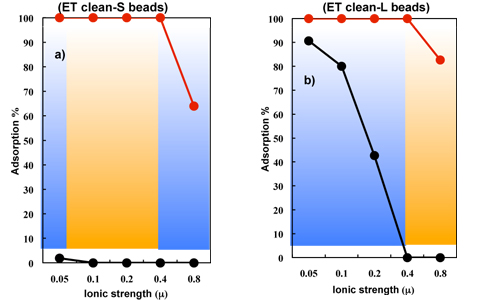
Selective adsorption of endotoxin was determined using a batchwise method with 0.2 g of the wet beads and 2 ml of a sample solution (BSA: 500 μg/ml, E. coli O111: B4 LPS: 100 ng/ml, pH 7.0, ionic strength of μ = 0.05-0.8 ).
Selective removal of endotoxin from a protein solution by Cellufine™ ET clean beads.
| Sample Solution | Cellufine™ ET clean S | Cellufine™ ET clean L | |||
|---|---|---|---|---|---|
| Compound(pI) | Concentration of endotoxin before treatment(pg/ml) | (0.02M PB, pH7.0, μ=0.05) | (0.02M PB + 0.36M NaCl, pH7.0, μ=0.40) | ||
| Concentration of endotoxin after treatment(pg/ml) | Recovery of protein after treatment(%) | Concentration of endotoxin after treatment(pg/ml) | Recovery of protein after treatment(%) | ||
| Ovalbumin(4.6) | 28,000 | 81 | 99 | <10 | 95 |
| BSA(4.9) | 32,000 | 45 | 99 | <10 | 97 |
| Myoglobin(6.8) | 4,500 | 18 | 99 | <10 | 98 |
| γ-globulin(7.4) | 5,600 | 20 | 99 | <10 | 97 |
| Cytochrome C(10.6) | 1,500 | 15 | 99 | <10 | 98 |
2 ml of protein solution (1 mg/ml, LPS: contaminant in various protein reagents) was added to 0.3 mL of ET clean. 1 endotoxin unit (EU) = 250pg LPS can be converted.
Application Data
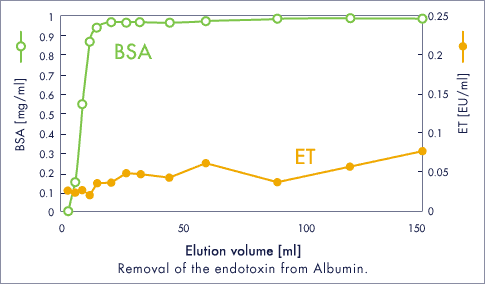
ET removal Exp.
BSA / ETclean L
Column chromatography
- Column size
- 1 X 1.1 cm (I.D.) (1.1ml)
- Flow rate
- 0.17 ml / min (10cm / h)
- Buffer
- 50 mM PB, pH 7 + 0.15 mol NaCl aq
Assay
- Protein Abs. at 280 nm
- ET LAL rate assay
- Injection sample (150 ml)
- BSA 1 mg/ml ET 100 EU/ml
Lysozyme / ET clean L
Column chromatography
- Column size
- 10 x 0.9 cm (I.D.) (9.6 ml)
- Flow rate
- 0.5 ml / min (47 cm / h)
- Buffer
- 1 mM Tris-HCl, pH 7.3
- Gradient
- 0 → 1.0 mol / l NaCl aq.
Assay
- Protein Abs. at 280 nm
- ET LAL rate assay
- Injection sample (1ml) : 14 mg / ml
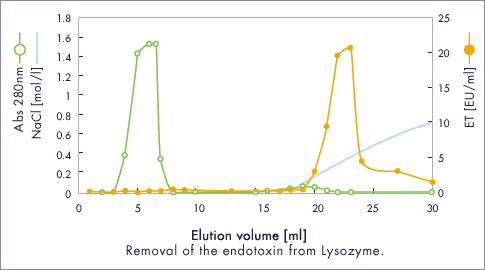
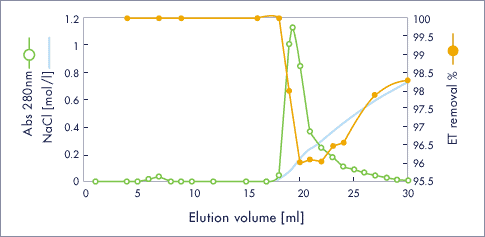
Insulin chain A / ET clean L
- Injection sample (1ml) : 13 mg / ml, 309 EU / ml
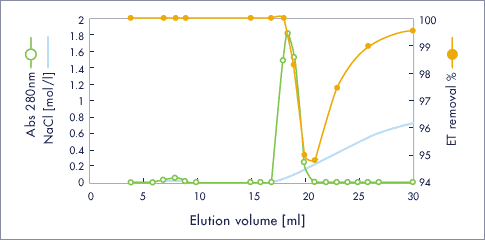
Tranceferrin / ET clean L
- Injection sample (1ml) : 13 mg / ml, 2982 EU / ml
References
1) M. Sakata, M. Todokoro, C. Hirayama, American Biotechnol. Lab., 20 (2002) 36.
2) M. Todokoro, M. Sakata, S. Matama, M. Kunitake, J. Ohkuma, C. Hirayama, J. Liq. Chrom. & Rel. Technol., 25 (2002) 601.
- Cellufine ET clean S
- Cellufine ET clean L
