CellufineTM MAX S, Q, CM, DEAE, GS
High Flow Rate, High Binding Capacity
Cellufine™ MAX is the new, high-flow, Cellufine media. JNC’s advanced cross-linking technologies have created more robust base beads operable at high flow and pressure. Further, Cellufine MAX ion exchange (IEX) media are made using surface modification techniques that dramatically increase ligand availability, which translates to higher dynamic binding capacities. Cellufine MAX IEX media are offered in six products, including both anion and cation chemistries.
Cellufine MAX Base Resin
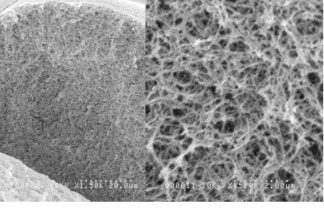
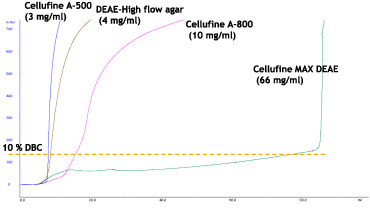
Cellulose, natural polysaccharide, possesses unique crystalline molecular structure differing from non-crystalline polysaccharides such as agarose. Thus Cellufine has unique pore structure as shown in the pictograph (Fig. 1). The new Cellufine MAX series offers the largest pore size of all Cellufine chromatography media. The benefit of such pore size in Cellufine MAX IEX media provides superior strength and excellent mass transfer. This is seen in the break-through curves for thyroglobulin, a very large protein (Fig. 2).
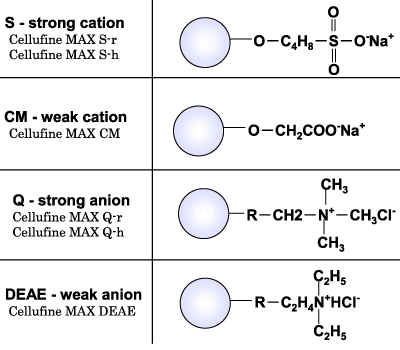
Partial Structure of Cellufine MAX IEX Media
Ligand structure for Cellufine MAX IEX media are described in Fig. 3. S, Q, CM and DEAE are correspondingly strong cation, strong anion, weak cation and weak anion exchangers. Two sub-types, h and r, are available for Cellufine MAX S and Q.
The differences between X-h and X-r type Cellufine MAX strong ion exchange media (X) are due to the design of the media. The X-h type is designed for higher binding capacity than the X-r type by optimizing the ligand content and dextran scaffold.
Characteristics of Cellufine MAX IEX Media
The basic characteristics of Cellufine MAX IEX media are shown in Table 1. All Cellufine MAX IEX media are based on 90 μm (average) highly cross-linked cellulose beads, which are surface-modified with dextran. Cellufine MAX IEX media are designed for use in bio-pharmaceuticals purification processes.
| Type | MAX CM | MAX S-r | MAX S-h | MAX DEAE | MAX Q-r | MAX Q-h | |
|---|---|---|---|---|---|---|---|
| Matrix | Cross-linked cellulose with dextran scaffold | ||||||
| Particle size (μm) | 40 - 130 | ||||||
| Ligand | CM | S | S | DEAE | Q | Q | |
| Ion exchange capacity (meq / ml-gel) | 0.09 - 0.22 | 0.09 - 0.21 | 0.10 - 0.22 | 0.12 - 0.22 | 0.10 - 0.20 | 0.13 - 0.22 | |
| 10% DBC(mg/ml) | Lysozyme | 220 | 144 | 191 | |||
| BSA | 197 | 141 | 225 | ||||
| human-γ-globulin | 104 | 131 | 216 | 108 | 74 | 135 | |
| pH stability | 2 -13 | 2 -13 | 3 -14 | 2 -12 | 2 -12 | 2 -12 | |
| Storage | 20% Ethanol | ||||||
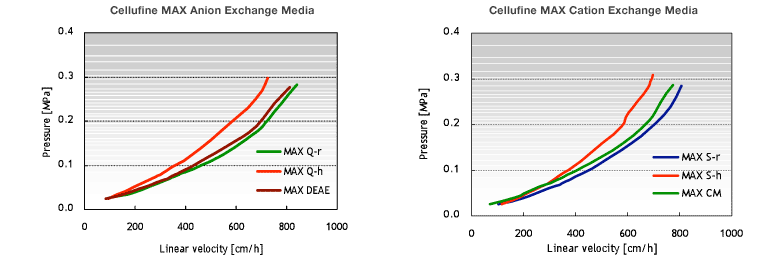
Pressure-flow Properties of Cellufine MAX IEX Media
Cellufine MAX IEX media enable high-flow operation, which is essential to efficient purification of bio-pharmaceuticals.
The figures below show pressure-flow velocity curves of Cellufine MAX IEX media in a 30 cm column with a 20 cm bed height (Fig. 4). All Cellufine MAX IEX media are operable at practical flow velocities (500 cm/h) and pressures.
- Column
- 30 cm I.D. x 20 cm L
- Mobile phase
- Pure Water at 24 ºC
Dynamic Binding Capacities of Cellufine MAX IEX Media
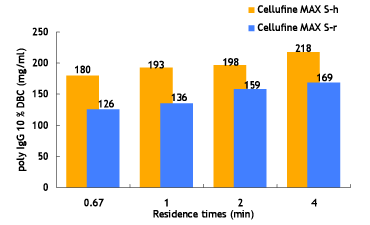
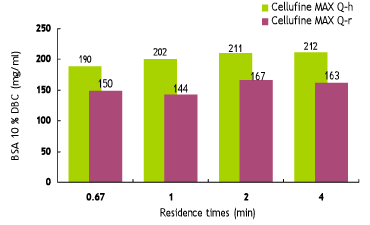
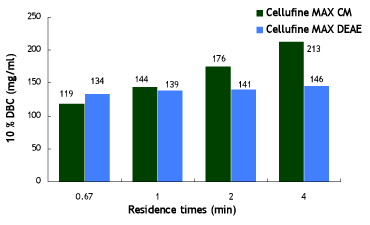
Efficient mass-transfer characteristics of Cellufine MAX IEX media translate to superior dynamic binding capacities (DBC). Figure 5 to 7 show DBC of model proteins at different residence times for Cellufine MAX IEX media. All Cellufine MAX IEX media are stable over a range of residence times.
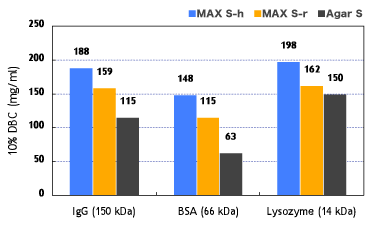
Fig. 8 shows that Cellufine MAX S exhibits superior dynamic binding performance across a range of protein characteristics to competitive media.
These unique characteristics of Cellufine MAX IEX media make it suitable for use in up-stream as well as to down-stream steps in bio-pharmaceuticals purification.
Cellufine MAX Cation Exchange Media
- Column
- 5 mm ID×50 mm L
- Sample
- human polyclonal IgG (1 mg/ml)
- Buffer
- 10 mM Acetate-50 mM NaCl (pH 4.3)
Cellufine MAX Anion Exchange Media
- Column
- 5 mm I.D. x 100 mm L
- Sample
- BSA (1 mg/ml)
- Buffer
- 50 mM Tris-HCl (pH 8.5)
Cellufine MAX Weak ion Exchange Media
- Column
- 5 mm ID x 50 mm L
- Sample
- human polyclonal IgG (1 mg/ml)
BSA (1 mg/ml) - Buffer
- 10 mM Acetate (pH 5.6) for IgG
Tris-HCl (pH 8.5) for BSA
Cellufine MAX Cation Exchange Media
- Polyclonal IgG
- 10 mM Acetate (pH 4.3) - 50 mM NaCl
- BSA
- 10 mM Acetate (pH 4.3) - 50 mM NaCl
- Lysozyme
- Tris-HCl (pH 9.5)
Model Proteins Separation Performance for Cellufine MAX IEX Media
Cellufine MAX IEX media are optimized for high adsorption and high resolution. Model protein separation with MAX S-h and MAX CM (Strong Cation vs. Weak Cation) is demonstrated in Fig. 9 and 10.
Cellufine MAX Cation Exchange Media
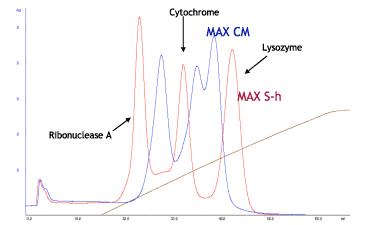
- Column
- 6.6 mm ID×50 mm L
- Buffer A
- 10 mM phosphate buffer (pH 7)
- Buffer B
- 10 mM phosphate (pH 7) + 1 M NaCl
(0→50 % linear gradient) - Flow rate
- 0.86 ml/min (residence time: 2min)
- Proteins
- Ribonuclease A (5 mg/ml),
Cytochrome C (2.5 mg/ml),
Lysozyme (1.5 mg/ml) - Injection volume
- 1.5ml
Cellufine MAX Anion Exchange Media
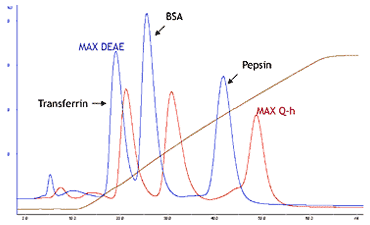
- Column
- 6.6 mm ID×50 mm L
- Buffer A
- 50 mM Tris-HCl (pH 8.5)
- Buffer B
- 50 mM Tris-HCl (pH 8.5) - 1 M NaCl
(0→75 % linear gradient) - Flow rate
- 0.86 ml/min (residence times 2 min)
- Proteins
- Transferrin (5 mg/ml),
BSA (10 mg/ml),
Pepsin (5 mg/ml) - Injection volume
- 1.5 ml
Chemical Stability and Cleaning-In-Place
Cellulose is well-known as a natural product having chemical and physical stability. Thus, since Cellufine is derived from cellulose, it also is stable to chemicals, caustic and acidic solutions. CIP of all Cellufine MAX IEX media can be carried out with 0.5 M NaOH solution. Used media should be stored in 20 % ethanol at 2 - 25 ºC after cleaning.
Cellufine MAX GS has been developed as a new strong cation chromatography media, with optimized ligand density. Cellufine MAX GS shows a superior performance for aggregate removal from therapeutic Mabs.
Characteristics of Cellufine MAX GS Media
The basic characteristics of Cellufine MAX GS are shown in Table 1. The base matrix for Cellufine MAX GS is 90 μm (average) highly cross-linked cellulose beads, the same as other Cellufine MAX IEX media.
| Matrix | Highly Cross-linked Cellulose |
|---|---|
| Particle size | 40~130 μm |
| Ligand type | -R-SO3-Na+ |
| Ion Exchange Capacity (m mol / ml) | 0.09〜0.15 |
| Lysozyme adsorption capacity (mg / ml) | ≧ 100 |
| Polyclonal IgG 10% DBC (mg / ml) | ≧ 70 |
| Operating pressure | < 0.3 MPa |
| pH stability | pH 2 ~ 13 |
Pressure-Flow Properties of Cellufine MAX GS
Figure 1 shows pressure-flow velocity curves of Cellufine MAX GS in 30 cm I.D. column with 20 cm bed height. Cellufine MAX GS has excellent flow properties and is applicable for process production.
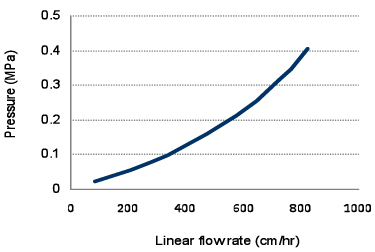
- Column
- 30 cm I.D. x 20 cm L
- Mobile Phase
- Pure water (24℃)
Model Protein Separation Performance for Cellufine MAX GS
Cellufine MAX GS is highly effective aggregates from IgG monomer by NaCl or pH gradient. Figure 2 shows the comparison of Cellufine MAX GS with S agarose media for monomer /aggregate separation of polyclonal IgG (Figure 2a) and monoclonal antibody (Figure 2b) by NaCl gradient elution. Cellufine MAX GS has been shown to be applicable an effective media for aggregate removal from Mabs.
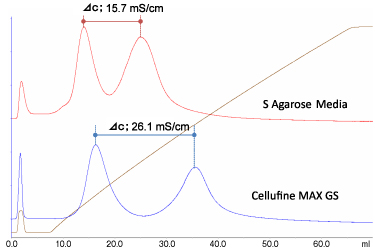
- Poly IgG
- Thermal and acid stressed Poly IgG
- Buffer
- Acetate (pH5.0), 50mM →1 M NaCl Poly IgG
- injection
- 1 ml
- Poly IgG conc
- 2 mg/ml
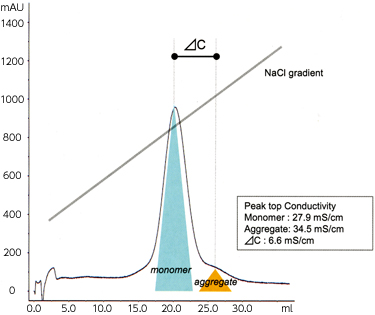
- Column
- 5 mm ID × 50 mm L
- Buffer
- Citrate Buffer (pH 5.0)
- NaCl Gradient
- 0.2→0.5 M
- Mab Injection
- 1 ml
- Flow rate
- 0.66 ml/min
Dynamic Binding Capacities of Cellufine MAX GS
Efficient mass-transfer characteristics of Cellufine MAX GS translate to superior dynamic binding capacities (DBC). Figure 3 shows DBC of Poly IgG at various residence times. Cellufine MAX GS is suitable for use in down-stream steps in antibody purification.
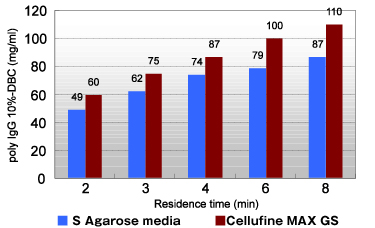
- Conditions
- Column
- φ5 mm×5 cm L
- Poly_IgG Concentration
- 1 mg/ml
- Adsorption Buffer
- 10 mM Acetate (pH 5.0) + 50 mM NaCl
- Cellufine MAX S-r , S-h
- Cellufine MAX GS
- Cellufine MAX Q-r , Q-h
- Cellufine MAX CM
- Cellufine MAX DEAE
